Alnylam Stock Up on Cardiomyopathy Drug Regulatory Filing in the EU
Alnylam Pharmaceuticals (NASDAQ: ALNY) announced that it has submitted a Type II Variation to the European Medicines Agency for vutrisiran, an investigational RNAi therapeutic being developed to treat ATTR amyloidosis with cardiomyopathy (ATTR-CM). Shares of the company gained 4.6% in response to the encouraging news.
Please note that Alnylam already markets vutrisiran in the EU under the brand name Amvuttra for treating hereditary transthyretin-mediated (hATTR) amyloidosis in adult patients with stage 1 or stage 2 polyneuropathy.
The application seeking approval for vutrisiran to treat ATTR-CM is supported by positive results from ALNY’s pivotal phase III HELIOS-B study, which met all primary and secondary endpoints across both the overall and monotherapy populations, each with statistical significance.
Year to date, shares of Alnylam have gained 57% against the industry’s 1% decline.
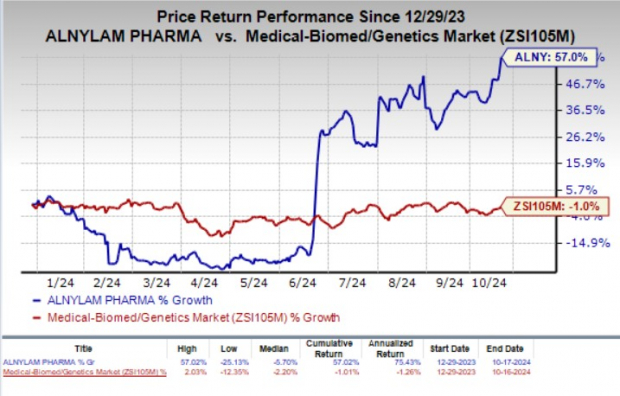
Image Source: Zacks Investment Research
Per the data readout, vutrisiran reduced mortality and cardiovascular events while improving functional capacity (measured by the six-minute walk test), quality of life (using the Kansas City Cardiomyopathy Questionnaire), and heart failure symptoms and severity (via New York Heart Association class) in ATTR-CM patients.
Additionally, the safety profile of vutrisiran in the HELIOS-B study was consistent with its known profile for treating hATTR amyloidosis with polyneuropathy in adults. In the late-stage study, the rates of adverse events, including those leading to discontinuation, were similar between the vutrisiran and …
Full story available on Benzinga.com